
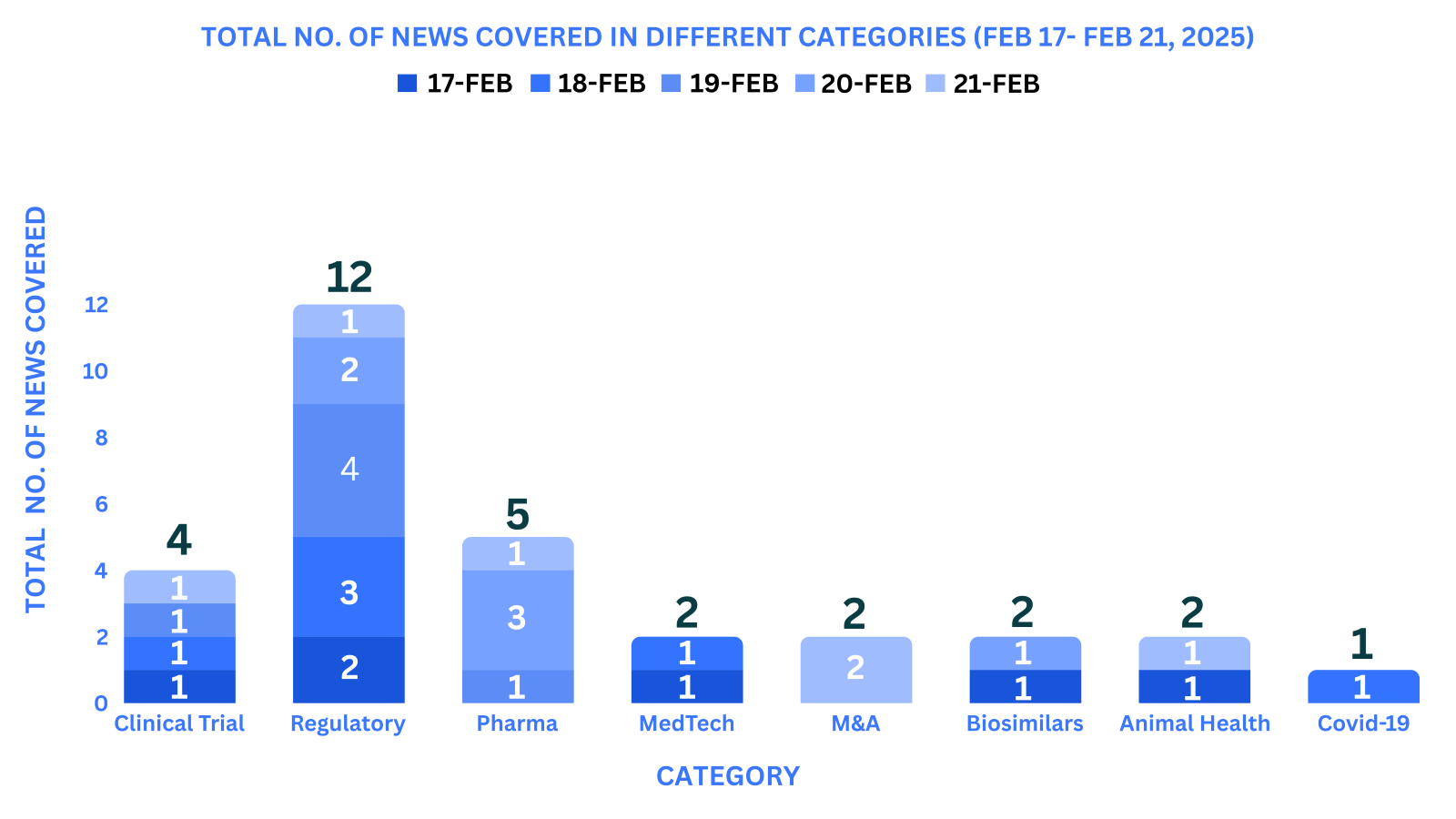
PharmaShots Weekly Snapshots (February 17th, 2025 – February 21th, 2025)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, M&A, Animal Health, COVID 19 & Biosimilars. Check out our full report below:

AstraZeneca Presents Post-Hoc Data from P-III (NIAGARA) Trial of Imfinzi in Muscle-Invasive Bladder Cancer Patients regardless of Complete Pathology Response at ASCO GU 2025
Read More: AstraZeneca
Exelixis Presents 5-year Follow-Up Data from P-III (CheckMate -9ER) of Cabometyx + Opdivo in Renal Cell Carcinoma Patients at ASCO GU 2025
Read More: Exelixis
BMS Reports P-III (CheckMate-816) Trial Data of Opdivo as a Neoadjuvant Treatment of Resectable NSCLC
Read More: BMS
Akeso Reports First Patient Enrollment in P-III Study of Ivonescimab + CT to Treat Triple-Negative Breast Cancer (TNBC) in China
Read More: Akeso

Ono Pharmaceutical Reports the US FDA Approval of Romvimza to Treat Symptomatic Tenosynovial Giant Cell Tumor (TGCT) in Adults
Read More: Ono Pharmaceutical
GSK Reports the US FDA Approval of Penmenvy (MenABCWY Vaccine) for Protection Against Invasive Meningococcal Disease (IMD)
Read More: GSK
Galderma’ Nemluvio Receives UK’s MHRA & Swissmedic Approval for Atopic Dermatitis and Prurigo Nodularis
Read More: Galderma
Regeneron and Sanofi Report the US FDA’s sBLA Acceptance for Dupixent to Treat Bullous Pemphigoid (BP)
Read More: Regeneron and Sanofi
The US FDA Grants Breakthrough Therapy Designation to Innate Pharma’s Lacutamab for R/R Sézary Syndrome
Read More: Innate Pharma
Merus Receives the US FDA’s Breakthrough Therapy Designation for Petosemtamab to Treat 1L PD-L1+ Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (R/M HNSCC)
Read More: Merus
Merck Receives the EC’s Approval for Welireg (Belzutifan) for Von Hippel-Lindau (VHL) Disease-Associated Tumors and Previously Treated Renal Cell Carcinoma
Read More: Merck
Gilead Reports US FDA’s NDA Acceptance of Lenacapavir for Pre-Exposure Prophylaxis (PreP) to Prevent HIV in Individuals at Risk
Read More: Gilead
The US FDA Accepts NDA of Boehringer Ingelheim’s Zongertinib for HER2 (ERBB2)-Mutant NSCLC
Read More: Boehringer Ingelheim
GSK Reports the China’s NMPA Acceptance of Nucala (Mepolizumab) as an Add-on Treatment for COPD
Read More: GSK
Gilead Receives EC’s Conditional Marketing for Seladelpar to Treat Primary Biliary Cholangitis (PBC)
Read More: Gilead

Biogen Collaborates with Stoke Therapeutics to Develop and Commercialize Zorevunersen for Dravet Syndrome
Read More: Biogen and Stoke Therapeutics
Epitopea Partners with Merck to Discover Cryptigen Tumor-Specific Antigens Using Epitopea’s CryptoMap platform
Read More: Epitopea and Merck
CSPC Pharmaceutical Collaborates with Radiance Biopharma to Develop and Commercialize RB-164 (SYS6005) as an Anti-Cancer Therapy
Read More: CSPC Pharmaceutical and Radiance Biopharma
Harbour BioMed Enters into a Collaboration with Insilico Medicine to Advance AI-Driven Antibody Discovery and Development
Read More: Harbour BioMed and Insilico Medicine
Incyte Partners with Genesis to Develop and Commercialize Novel Small Molecules Using Genesis’ GEMS AI Platform
Read More: Incyte and Genesis

Navi Medical Technologies Receives the US FDA’s 510(k) Clearance for Neonav ECG Tip Location System
Read More: Navi Medical Technologies
The US FDA Approves MED-EL’s Sonnet 3 Audio Processor for Optimal Hearing
Read More: MED-EL

Cosette Pharmaceuticals to Acquire Mayne Pharma for ~$430M
Read More: Cosette Pharmaceuticals and Mayne Pharma
AstraZeneca Purchases FibroGen China for ~$160M
Read More: AstraZeneca and FibroGen China

Anivive Lifesciences Completes Pivotal Field Study of Laverdia-CA1 (Verdinexor Tablets) for Canine Lymphoma
Read More: Anivive Lifesciences
Elanco Launches Pet Protect Supplement Line for Dogs and Cats
Read More: Elanco

Samsung Bioepis Reports the EC’s approval of Obodence & Xbryk (Biosimilar, Denosumab)
Read More: Samsung Bioepis
Celltrion Secures the EC’s Approval for Eydenzelt (Biosimilar, Eylea), Stoboclo & Osenvelt (Biosimilars, Prolia & Xgeva)
Read More: Celltrion
Alvotech and Teva Report the Regulatory Filing Acceptance for AVT06 (Biosimilar, Eylea) Across the US
Read More: Alvotech and Teva

CSL and Arcturus Therapeutics Report the EC’s Approval of Kostaive Against COVID-19
Read More: CSL and Arcturus Therapeutics
Related Post: PharmaShots Weekly Snapshots (February 10th, 2025 – February 14th, 2025)
Tags

Ridhi is an avid secondary researcher who follows trends in the biopharmaceutical and healthcare sectors to curate engaging content for the global audience. She works as a news editor at PharmaShots and loves to read books and explore new destinations.














